Fresh Opportunity for the Sodium–Sulfur Battery
Over 50 years ago, the sodium–sulfur battery was considered promising, but it failed to make its big breakthrough. Its poor performance at room temperature is a disadvantage. A Jülich development might now be able to solve this problem.
Sodium–sulfur batteries made their first major appearance in 1966 when automobile manufacturer Ford presented the battery to experts as a possible power source for electric cars. The batteries featured heat-resistant housings and were operated at temperatures of above 330 °C. Twenty-seven years later, Ford installed a battery of this type in vehicles for the first time. However, after several years of tests with 100 prototypes of the small Ford Ecostar delivery van, during which batteries burst into flames several times, the company made the switch to fuel cell technology. The US Air Force and the Netherlands Marine Corps also investigated the application of sodium–sulfur batteries in space travel and in submarines, but this did not lead to series production. Things then went quiet on this battery type. Only one Japanese company currently produces such batteries with up to 1.45 megawatt hours for stationary applications, which are sold by a large German chemical company, among others.
However, over the last ten years, research into sodium–sulfur batteries has regained significance. Researchers worldwide are looking for alternatives to the lithium-ion batteries that currently dominate the market, as lithium is expensive and it has come under criticism due to the negative environmental impacts of lithium mining. The same applies for the metal cobalt, which is used in many lithium-ion batteries.
In contrast, sodium is available in large quantities and is more environmentally friendly to extract than lithium, which also makes it much more cost-efficient. However, sodium-based batteries are heavier and require more space to store the same amount of energy as a lithium-ion battery. “With stationary storage systems for wind and solar power, weight and space requirements do not play as big a role as they do for batteries for electric vehicles or laptops,” explains Dr. Frank Tietz from Forschungszentrum Jülich’s Institute of Energy and Climate Research (IEK-1). “In such cases, cost is the more decisive factor for the storage systems.”
High temperatures required so far
There is one factor, however, complicating the economic viability of sodium-sulfur batteries: they only work to a satisfactory level at temperatures of above 250 °C; at lower temperatures, too few charge carriers migrate from one pole to the other (see image below).
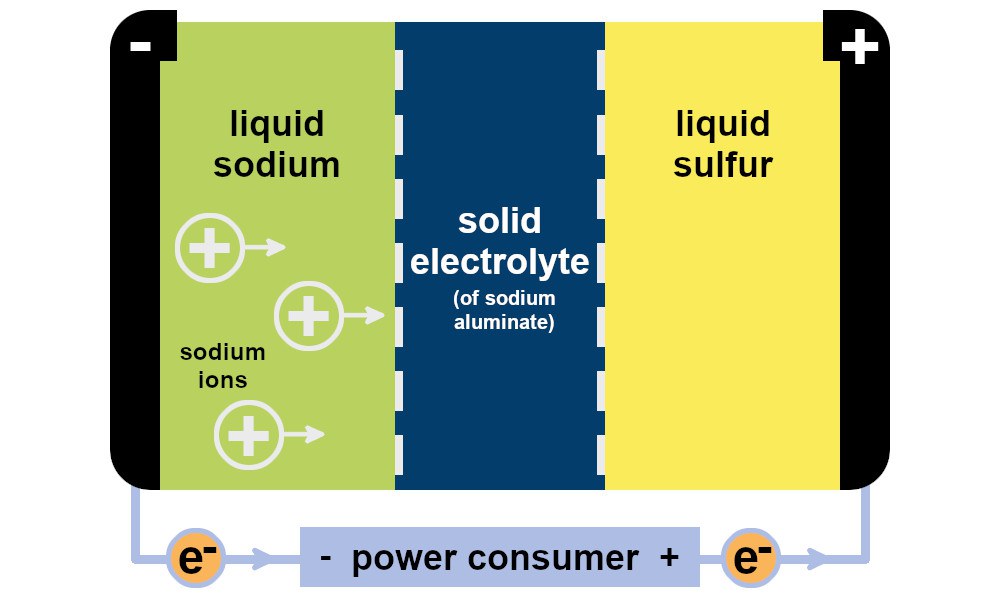
In these high-temperature batteries, liquid sodium works as an anode and liquid sulfur in a graphite fabric as the cathode. They are separated by a solid electrolyte consisting of a sodium aluminate layer. If the operating temperature is lowered, not enough charge carriers – sodium ions – pass through the electrolyte from one electrode to the other. This is because the sodium and sulfur electrodes are then no longer molten but are in solid form, which makes the migration or penetration of ions more difficult. In addition, contact with the electrolyte deteriorates, as solid surfaces are then adjacent to each other and the electrolyte is no longer wetted by the molten electrodes.
Dr. Tietz’s team has found an approach to ensure that sufficient charge carriers are also able to migrate at room temperature. To do so, the researchers made two adjustments. The decisive change they made was a considerable reduction in the thickness of the electrolyte. “The area-specific resistance of the solid electrolyte decreases tenfold if it is only a tenth as thick as usual,” explains Dr. Tietz. The researchers also optimized the contact possibilities between the electrolyte and the two poles of sodium and sulfur, respectively. Instead of producing the electrolyte from a single layer as usual, the Jülich researchers constructed it like a sandwich consisting of three layers: a more stable, dense middle layer surrounded by two porous layers. Sodium and sulfur can be stored within these layers, which improves the contact between the electrolyte and the electrode materials and, in turn, the energy density of the battery.
Energy density with potential
“Although there is still room for improvement, particularly on the sulfur electrode side, we have already achieved an energy density of around 46 hours (Wh) per kilogram. A value of around 280 Wh per kilogram would theoretically be possible with this cell structure,” says Aikai Yang, a doctoral researcher from China who developed the prototype (see box). By comparison, current lithium-ion batteries have an energy density of between 100 Wh and 250 Wh per kilogram.
“We will continue to research this promising battery type, partly with the aim of producing larger battery cells. These could then be connected to each other to form cost-effective stationary battery storage systems,” says Prof. Olivier Guillon, director at IEK-1. According to the materials scientist, the new solid electrolyte has already attracted interest from industry. This might help the sodium-sulfur battery to make the most of its new opportunity.
Sandwich electrolyte
The prototype of the solid-state battery has a diameter of one centimetre. Doctoral researchers Aikai Yang and Ruijie Ye developed the core of the battery, the electrolyte. The challenge was to produce an ion-conducting ceramic that is less than half a millimetre thick, yet does not break and consists of three defined layers. Yang found that a sodium yttrium silicate is suitable for this purpose. Ye used the tape casting method to construct the three layers. He processed a mixture of finely ground ceramic powder with water, a deflocculant, and a binder to form a kind of paste known as a slip. To produce the two porous ceramic layers, he added acrylic glass spheres to the mixture. Using a special system, he distributed the slip evenly on a carrier tape. After the liquid components had evaporated, a uniform and thin ceramic layer was formed. This layer was combined with the other two layers by laminating, pressing and heating to form a solid electrolyte.

Text: Frank Frick | images: Forschungszentrum Jülich/Sascha Kreklau; Forschungszentrum Jülich/Tobias Schlößer | graphics: Forschungszentrum Jülich
Original publications:
Yang A, Ye R, Song H, et al. Pressureless all-solid-state Na/S batteries with self-supporting Na5YSi4O12 scaffolds. Carbon Energy. 2023; 5:e371. doi:10.1002/cey2.371
Yang A, Ye R, Li X, et al. Fabrication of thin sheets of the sodium superionic conductor Na5YSi4O12 with tape casting. Chem Eng J. 2022; 435 (Part 1):134774. https://doi.org/10.1016/j.cej.2022.134774